肺内リンパ球、マクロファージ数の増加とサイトカイン発現を誘導し、病態の軽減に有効である
Retinoic Acid Therapy Attenuates the Severity of Tuberculosis While Altering Lymphocyte and Macrophage Numbers and Cytokine Expression in Rats Infected with Mycobacterium tuberculosis.
Hiroyuki Yamada, Satoru Mizuno, A. Catharine Ross, and Isamu Sugawara
Journal of Nutrition, 137: 2696-2700, 2007. に掲載された論文の要旨と図表を掲載したものです。
【目的】ビタミンAの誘導体の一つで生理活性が高いレチノイン酸(RA)は、マクロファージを刺激し、一方、結核菌は肺胞マクロファージを標的として感染することから、ラットの結核感染におけるRAの治療的効果を検討した。
【材料と方法】Wister-Lewisラット15匹を結核菌H37Rvで吸入感染し、体重100gあたり100μgのRAを食物油に溶解して週3日、3-5週間経口投与し、完了後解剖し、肺および脾臓内生菌数、組織内サイトカインmRNAの定量(real-time PCR)、病理組織像の検討を行った。
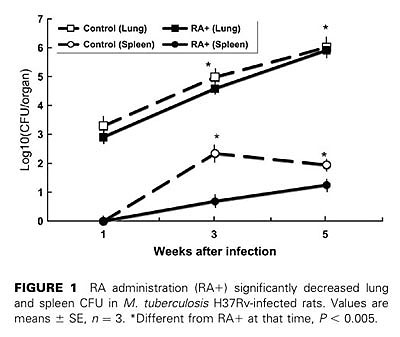
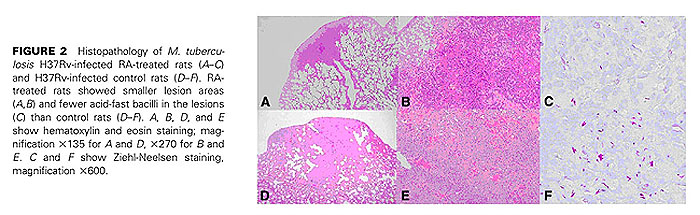
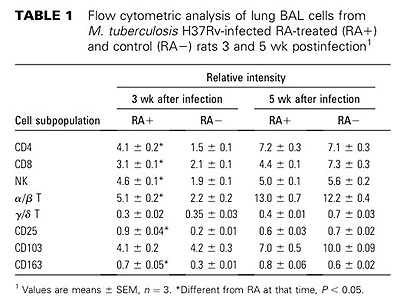
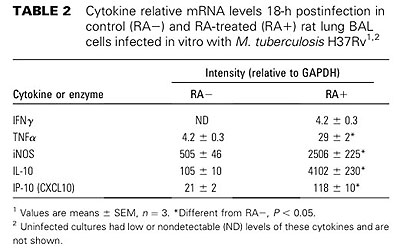
【結果と結論】RA投与群と対照群(食物油のみ投与)間で肺の組織像の上での病態に差が見られ、感染後3週、5週の肺および脾臓内生菌数はRA投与群で有意に減少していた(p<0.005)。感染肺組織内のCD4+, CD8+T細胞、NK細胞、CD163陽性マクロファージ数は、RA投与群で増加していた。また、RA投与群の感染肺組織におけるIFN-γとiNOS mRNAの発現量は対照群と比較して有意に多かった。更に、RA投与ラットからBALで得た肺胞マクロファージをin vitroで結核菌感染すると、対照群由来の肺胞マクロファージと比較して、IFN-γ, TNF-α, iNOS, IL-1β, IL-10, IP-10 mRNAの発現レベルが高かった。以上の結果から、RAの経口投与が結核菌のin vivo増殖を抑制し、病態の進展を押さえることが示唆された。
【文献】
- Villamor, E. and Fawzi, W.W. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 2005;18:446-64.
- Iwata, M., Eshima, Y., and Kagechika, H. Retinoic acids exert direct effects on T cells to suppress T(h)1 development and enhance T(h)2 development via retinoic acid receptors. Int. Immunol. 2003;15:1017-25.
- Iwata, M., Hirakiyama, A., Eshima, Y., Kagechika, H., Kato, C., and Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527-38.
- Ma, Y. and Ross, A.C. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc. Natl. Acad. Sci. USA. 2005;102:13556-61.
- Ma, Y., Chen, Q., and Ross, A.C. Retinoic acid and polyriboinosinic: polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J. Immunol. 2005;174:7961-9.
- Paterson, D.J., Jefferies, W.A., Green, J.R., Brandon, M.R., Corthesy, P., Puklavec, M., and Williams, A.F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol. Immunol. 1987;24:1281-90.
- Dawson, H.D., Li, N.Q., DeCicco, K.L., Nibert, J.A., and Ross, A.C. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J. Nutr. 1999;129:1510-7.
- Chen, Q. and Ross, A.C. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp. Cell. Res. 2004;297:68-81.
- Luo, X.M. and Ross, A.C. Physiological and receptor-selective retinoids modulate interferon gamma signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1. J. Biol .Chem. 2005;280:36228-36.
- Ross, A.C. and Harrison, E.H. Vitamin A: nutritional aspects of retinoids and carotenoids. In: Rucker RR, Zempleni J, McCormick DM, Suttie JW, editors. Handbook of vitamins. 4th ed. Boca Raton (FL): CRC Press; 2007. p. 1-40.
- Crowle, A.J. and Ross, E.J. Inhibition by retinoic acid of multiplication of virulent tubercle bacilli in cultured human macrophages. Infect. Immun. 1989;57:840-4.
- Anand, P.K. and Kaul, D. Vitamin D3-dependent pathway regulates TACO gene transcription. Biochem. Biophys. Res. Commun. 2003;310:876-7.
- Anand, P.K. and Kaul D. Downregulation of TACO gene transcription restricts mycobacterial entry/survival within human macrophages. FEMS Microbiol. Lett. 2005;250:137-44.
- Sugawara, I., Yamada, H., and Mizuno, S. Pathological and immunological profiles of rat tuberculosis. Int. J. Exp. Pathol. 2004;85:125-34.
- Yamada, H., Mizumo, S., Horai, R., Iwakura, Y., and Sugawara, I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab. Invest. 2000;80:759-67.
- Yamada, H., Udagawa, T., Mizuno, S., Hiramatsu, K., and Sugawara, I. Newly designed primer sets available for evaluating various cytokines and iNOS mRNA expression in guinea pig lung tissues by RT-PCR. Exp. Anim. 2005;54:163-72.
- Sugawara, I., Yamada, H., Kaneko, H., Mizuno, S., Takeda, K., and Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 1999;67:2585-9.
- Sugawara, I., Mizuno, S., Yamada, H., Matsumoto, M., and Akira, S. Disruption of nuclear factor-interleukin-6, a transcription factor, results in severe mycobacterial infection. Am. J. Pathol. 2001;158:361-6.
- Sugawara, I., Yamada, H., and Mizuno, S. Pathological and immunological profiles of rat tuberculosis. Int. J. Exp. Pathol. 2004;85:125?34.
- Luo, X.M. and Ross AC. Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1. Exp. Biol .Med. 2006;231:619-31.
- Yamada, H., Mizuno, S., and Sugawara, I. Interferon regulatory factor 1 in mycobacterial infection. Microbiol. Immunol. 2002;46:751-60.
updated 07/12/05